INTACT ACELLULAR FRAMEWORK
Retains native growth factors1,4
EFFECTIVE DECELLULARIZATION2,3
BIOHOSPITABLE MATRIX
Supports cell migration, cell proliferation, and vascularization5,6
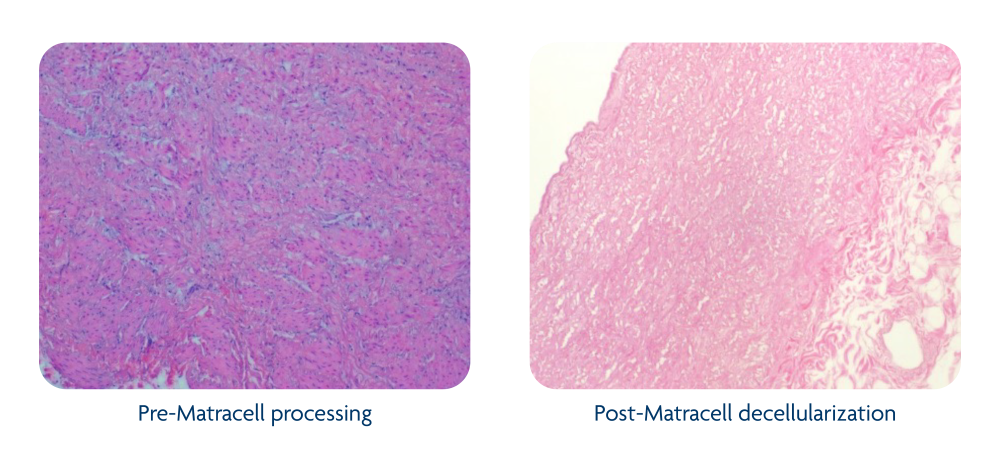
Decellularization of allograft tissue effectively removes donor cellular and DNA content that may elicit an immune response in the recipient, which can interfere with tissue healing. It has the added benefit of helping to remove microbial or viral contaminates that may also be present.1

The Matracell process is validated to thoroughly remove donor cellular and DNA content, including >90% DNA removal from placental membrane grafts, >97% DNA removal from dermal grafts, and >99% DNA removal from cardiovascular grafts.2,3,6

Matracell maintains essential extracellular matrix components, such as collagens, glycosaminoglycans, and elastin, to preserve its native biomechanics and support tissue remodeling.7-9

Dermal, placental, and some cardiovascular tissues processed with Matracell technology can be stored at ambient temperatures and are fully hydrated and ready to use—providing excellent handling and durability.10
Matracell's Two-Step Process

Decellularization using an anionic, non-denaturing detergent to solubilize donor cells combined with a recombinant endonuclease to degrade nuclear material.1-3,6

Rinsing to thoroughly remove the cellular content and reagent residuals and ensure biocompatibility.1-3,6
- Select Peer-Reviewed Publications
Decellularization of human dermis using non-denaturing anionic detergent and endonuclease: a review.
Moore MA, Samsell B, Wallis G, Triplett S, Chen S, Jones AL, Qin X. Cell Tissue Bank. 2015 Jun;16(2):249-59. doi: 10.1007/s10561-014-9467-4Properties of biologic scaffolds and their response to mesenchymal stem cells.
Beitzel K, McCarthy MB, Cote MP, Russell RP, Apostolakos J, Ramos DM, Kumbar SG, Imhoff AB, Arciero RA, Mazzocca AD. Arthroscopy. 2014 Mar;30(3):289-98. doi: 10.1016/j.arthro.2013.11.020Pulmonary arterioplasty with decellularized allogeneic patches.
Hopkins RA, Lofland GK, Marshall J, Connelly D, Acharya G, Dennis P, Stroup R, McFall C, O'Brien JE Jr. Ann Thorac Surg. 2014 Apr;97(4):1407-12. doi: 10.1016/j.athoracsur.2013.12.005Evaluation of host tissue integration, revascularization, and cellular infiltration within various dermal substrates.
Capito AE, Tholpady SS, AgrawalH, Drake DB, Katz AJ. Annals of Plastic Surgery. 2012. 68(5): 495-500. Doi: 10.1097/SAP.0b013e31823b6b01Macrophage phenotypes correspond with remodeling outcomes of various acellular dermal matrices.
Agrawal H, Tholpady SS, Capito AE, Drake DB, Katz KJ. Open Journal of Regenerative Medicine. 2012;1(3):51-59. Doi: 10.4236/ojrm.2012.13008An overview of tissue and whole organ decellularization processes.
Crapo PM, Gilbert TW & Badylak SF. Biomaterials. 2011 April;32(12):3233-3243. Doi: 10.1016/j.biomaterials.2011.01.057
References
1. Moore MA, Samsell B, Wallis G, Triplett S, Chen S, Jones AL, Qin X. Decellularization of human dermis using nondenaturing anionic detergent and endonuclease: a review. Cell Tissue Bank. 2015 Jun;16(2):249-59. doi: 10.1007/s10561-014-9467-4.
2. LifeNet Health, PQ-07-078, Process Qualification: Devitalization of Non-Valved Pulmonary Allograft
3. LifeNet Health, PQ-10-006, Process Qualification: Devitalization of Dermal Allografts
4. LifeNet Health, TR-004-2020, Technical Report: Characterization of the Amnion, Chorion, and Trophoblast Layers of Decellularized and Freeze-Dried Placental Membrane. 2020
5. Hopkins RA, Lofland GK, Marshall J, Connelly D, Acharya G, Dennis P, Stroup R, McFall C, O’Brien JE Jr. Pulmonary arterioplasty with decellularized allogeneic patches. Ann Thorac Surg. 2014 Apr;97(4):1407-12. Doi:10.1016/j.athoracsur.2013.12.005
6. LifeNet Health, PQ-11-005, Process Qualification: Biocompatibility of CardioGraft® with Matracell® XG
7. LifeNet Health, TR-0082
8. LifeNet Health, TR-0292, LifeNet Health’s Decellularized Dermis, Dermacell, Comparison to the USP Monograph, Scaffold Human Dermis.
9. LifeNet Health, 68-20-047, Analysis of the Acellular Matrix, Growth Factors, and Cytokines Present in Oracell.
10. LifeNet Health, PQ-10-008, Process Qualification: Method 2B Terminal Sterilization Validation for the Devitalized Dermis Allografts Family

