ArthroFlex SCR
Superior Capsular Reconstruction: Clinical Outcomes After Minimum 2-Year Follow-Up.
Hirahara AM, Andersen WJ, Panero AJ. AJO. 2017 Nov;46(6): 266-272, 278.
Superior Capsular Reconstruction: Clinical Outcomes After Minimum 2-Year Follow-Up.
Hirahara AM, Andersen WJ, Panero AJ. AJO. 2017 Nov;46(6): 266-272, 278.
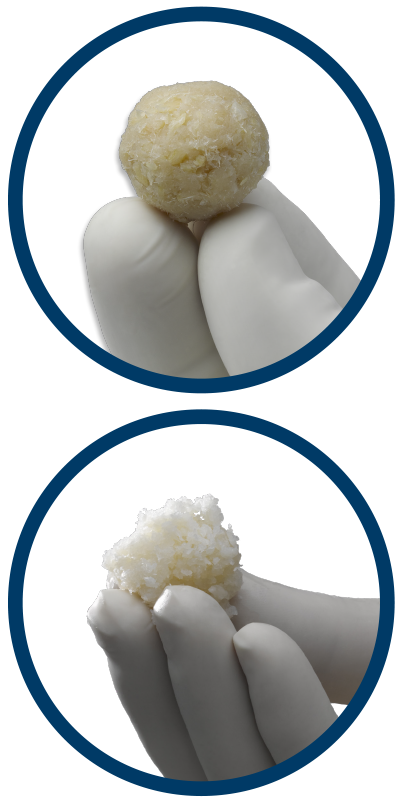
ViviGen represents a paradigm shift in the field of bone repair and tissue engineering by focusing on lineage committed bone cells. At every step of ViviGen’s processing, LifeNet Health has focused on maintaining the highest level of bone cell viability.

Many commercial DBMs utilize an inert carrier to improve handling, and the proportion of demineralized bone content in the graft varies widely by manufacturer.

Many commercial DBMs utilize an inert carrier to improve handling, and the proportion of demineralized bone content in the graft varies widely by manufacturer.
Wednesday, March 25, 2020
The Orlando Improv • Pointe Orlando
A non-CME medical education program to demonstrate improved clinical outcomes in complex shoulder procedures that include large to irreparable rotator cuff tears utizing ArthroFlex®, an acellular dermal matrix.
Attendees will have an opportunity to engage the speakers in discussions in a relaxed learning environment.
In this 2019 study, Dermacell AWM® was tested in its ability to heal complex diabetic foot ulcers with exposed tendons and/or bone. Deep wounds require advanced treatment because they are difficult to heal. They are rarely included in clinical trials for the study of effective options. This study tackles these complex wounds and finds that Dermacell AWM is effective at rapidly healing complex diabetic foot ulcers.
The clinical efficacy of Dermacell AWM is supported by numerous peer-reviewed and published articles in its intended field of use. Dermacell AWM is part of the largest human ADM in chronic wound randomized controlled trial to date, as well as findings by other peer-reviewed and published articles.
Dr. Zakee O. Shabazz, DPM, discusses his experience using Dermacell AWM when treating stalled healing diabetic foot ulcers. Dermacell AWM is a human acellular dermal matrix (ADM) that serves as a scaffold to reinforce damaged or inadequate soft tissue during wound healing. Dermacell AWM is decellularized using LifeNet Health's proprietary and validated Matracell® decellularization technology -- and is currently the only ADM that meets the definition of decellularization while preserving the extracellular matrix (ECM) that aids in the healing cascade.
The clinical efficacy of Dermacell AWM is supported by a growing number of peer-reviewed, clinical publications. The clinical efficacy of competing grafts has also been shown in the literature. However, information on cost-effectiveness of those grafts has been lacking. Using the most recent cost data, a meta-analysis determined that higher cost does not necessarily correlate with higher rates of healing. Dermacell AWM is an option that is not only clinically efficacious, but cost-effective too.