12 MILLION+ ALLOGRAFTS
distributed since 1995
0 REPORTS OF DISEASE TRANSMISSION
since 1995
MEDICAL DEVICE-GRADE STERILITY yielding a SAL of 10-6
First introduced in 1995, Allowash technology revolutionized the tissue banking industry, setting new standards for sterility while preserving the biomechanical and biochemical properties of allograft tissues.
Added in 2004, Allowash XG technology utilizes a validated low-dose gamma irradiation process administered at ultra-low temperatures in the final packaging, achieving a sterility assurance level (SAL) of 10-6.
Allowash XG addresses the potential for bioburden by virtually eliminating bacterial and viral risks to ensure the safest possible allografts while maintaining their clinical effectiveness.
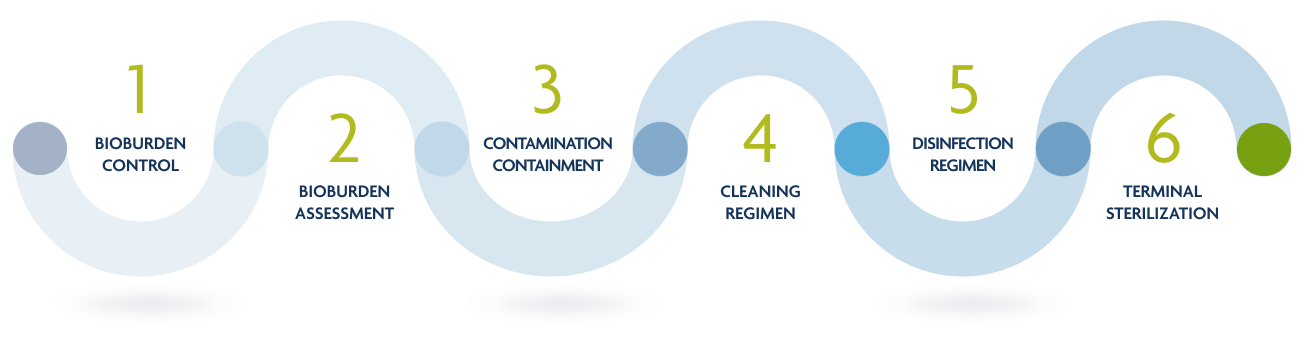
In addition to rigorous donor screening and advanced methods for aseptic recovery, Allowash XG includes refined methods for tissue processing with effective, yet gentle cleansing and disinfection that is tailored to the type of tissue. The most notable differences in tissue quality between providers often arise from the cleansing and disinfection phases.
LifeNet Health's unwavering commitment to patient safety and technological innovation has positioned Allowash XG as an indispensable advancement in the field of regenerative medicine, offering hope and healing to countless patients in need.
Allowash XG's Sterility Process